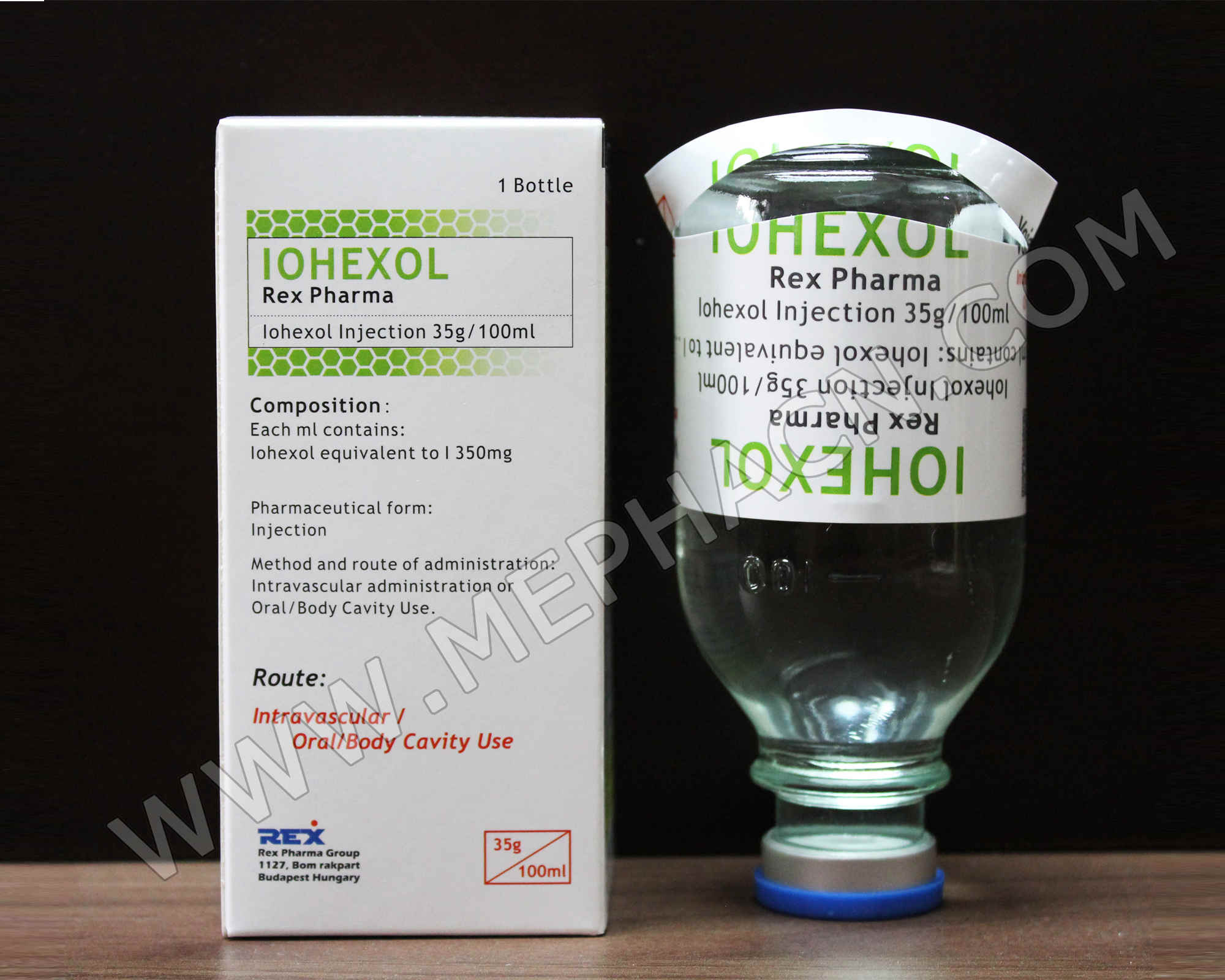
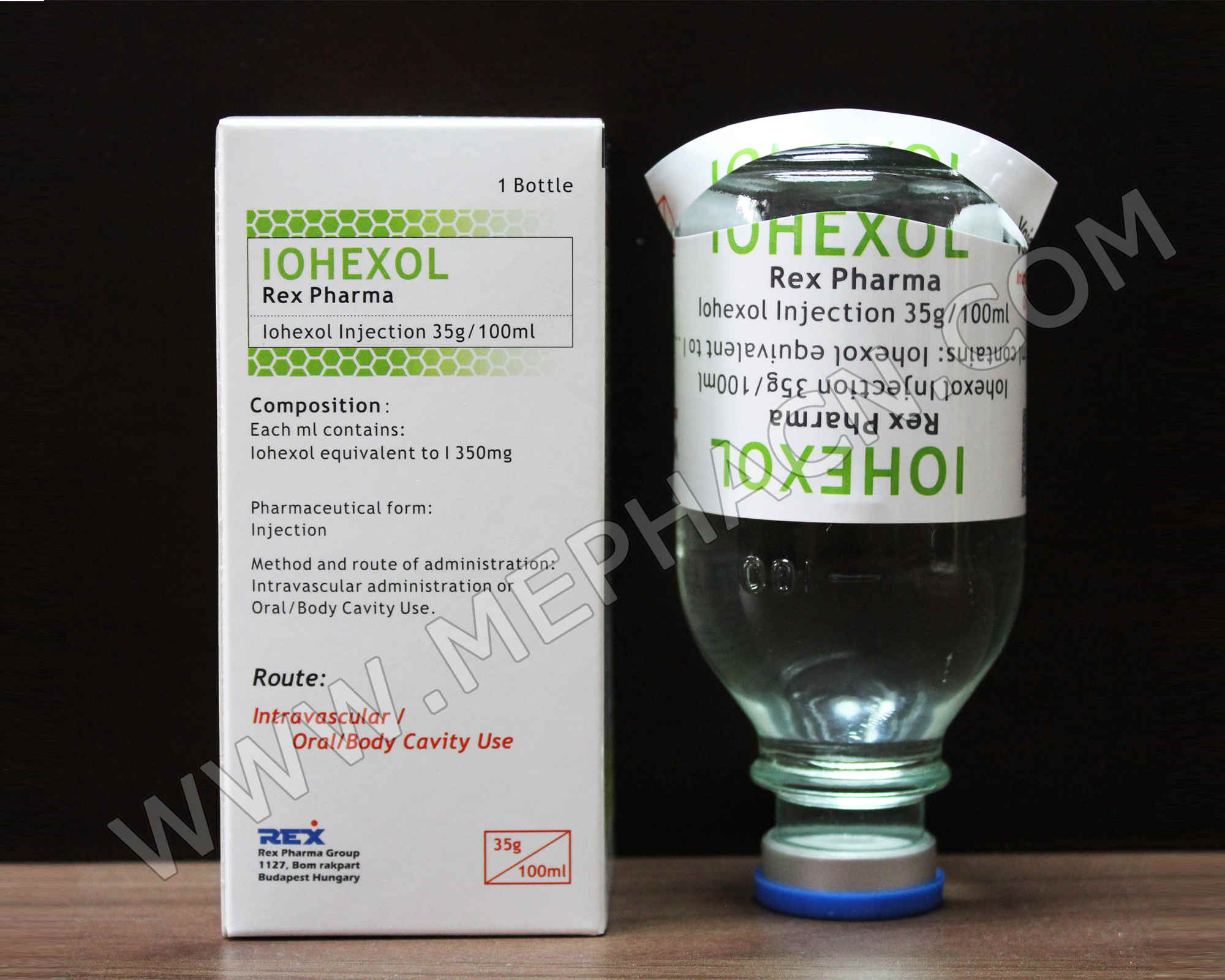
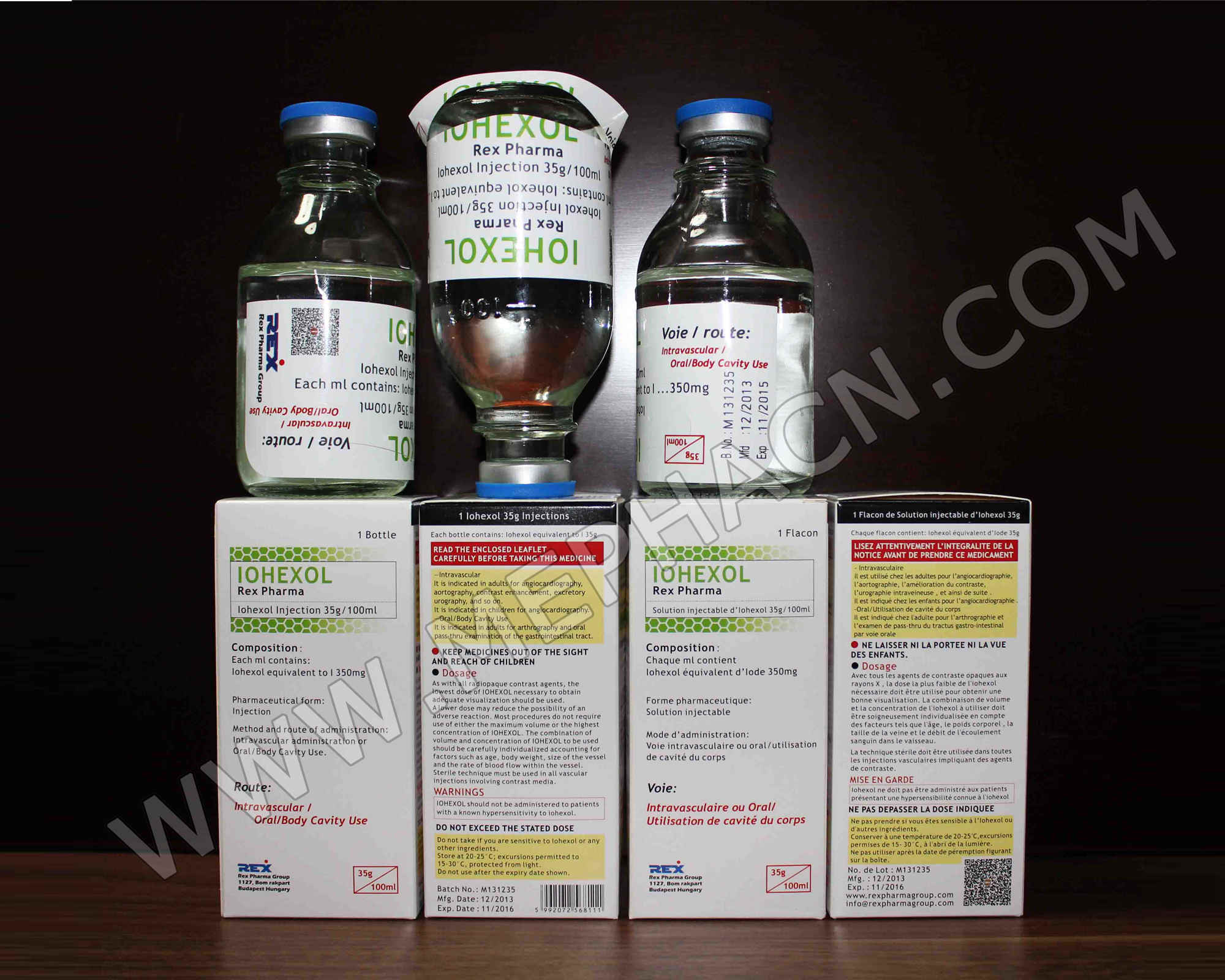
Iohexol infusion 35g/100ml, 100ml
1. Product name: Iohexol infusion
2. Specification: 35g/100ml
3. Package: 5 ampoules/tray/box
4. Shelf life: 36 months
5. Registration dossiers are available.
If you are interested in our products, please feel free to contact us.
1. What CITICOLINE-REX is and what it is used for
-Intravascular
IOHEXOL 350 is indicated in adults for angiocardiography (ventriculography, selective coronary
arteriography), aortography including studies of the aortic root, aortic arch, ascending aorta,
abdominal aorta and its branches, contrast enhancement for computed tomographic head and
body imaging, intravenous digital subtraction angiography of the head, neck, abdominal, renal and
peripheral vessels, peripheral arteriography, and excretory urography.
IOHEXOL 350 is indicated in children for angiocardiography (ventriculography, pulmonary
arteriography, and venography; studies of the collateral arteries and aortography, including the
aortic root, aortic arch, ascending and descending aorta).
-Oral/Body Cavity Use
IOHEXOL 350 have osmolalities from approximately 1.6 to 3.0 times that of plasma (285 mOsm/kg
water) and are hypertonic under conditions of use.
Adults: IOHEXOL 350 is indicated in adults for arthrography and oral pass-thru examination of the
gastrointestinal tract.
IOHEXOL diluted to concentrations from 50 mgI/mL to 100 mgI/mL is indicated in children for voiding
cystourethrography.
2. Before you use IOHEXOL-REX
CONTRAINDICATIONS
IOHEXOL should not be administered to patients with a known hypersensitivity to iohexol.
WARNINGS-General
Nonionic iodinated contrast media inhibit blood coagulation, in vitro, less than ionic contrast media.
Clotting has been reported when blood remains in contact with syringes containing nonionic contrast
media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been
reported during angiographic procedures with both ionic and nonionic contrast media.
PRECAUTIONS-General
Diagnostic procedures which involve the use of radiopaque diagnostic agents should be carried out
under the direction of personnel with the prerequisite training and with a thorough knowledge of
the particular procedure to be performed. Appropriate facilities should be available for coping with
any complication of the procedure,as well as for emergency treatment of severe reactions to the
contrast agent itself. After parenteral administration of a radiopaque agent, competent personnel
and emergency facilities should be available for at least 30 to 60 minutes since severe delayed
reactions have occurred .
3. How to use IOHEXOL-REX
As with all radiopaque contrast agents, the lowest dose of IOHEXOL necessary to obtain adequate
visualization should be used. A lower dose may reduce the possibility of an adverse reaction.
Most procedures do not require use of either the maximum volume or the highest concentration of
IOHEXOL . The combination of volume and concentration of IOHEXOL to be used should be carefully
individualized accounting for factors such as age, body weight, size of the vessel and the rate of blood
flow within the vessel. Other factors such as anticipated pathology, degree and extent of opacification
required, structure(s) or area to be examined, disease processes affecting the patient, and equipment
and technique to be employed should be considered.
Sterile technique must be used in all vascular injections involving contrast media.
Refer to DIRECTIONS FOR PROPER USE OF IOHEXOL PHARMACY BULK PACKAGE section for
instructions.
If nondisposable equipment is used, scrupulous care should be taken to prevent residual
contamination with traces of cleansing agents.
It may be desirable that solutions of radiopaque diagnostic agents be used at body temperature when
injected.
Parenteral products should be inspected visually for particulate matter and discoloration prior to
administration whenever solution and container permit. Solutions of IOHEXOL should be used only
if clear and within the normal colorless to pale yellow range. If particulate matter or discoloration is
present, do not use.
INDIVIDUAL INDICATIONS AND USAGE
-Intravascular
ANGIOCARDIOGRAPHY
AORTOGRAPHY AND SELECTIVE VISCERAL ARTERIOGRAPHY
IOHEXOL 350 at a concentration of 350 mgI/mL are indicated in adults for use in aortography and
selective visceral arteriography including studies of the aortic arch, ascending aorta, and abdominal
aorta and its branches (celiac, mesenteric, renal, hepatic and splenic arteries).
IOHEXOL 350 at a concentration of 350 mgI/mL is indicated in children for use in aortography
including studies of the aortic root, aortic arch, ascending and descending aorta.
CT SCANNING OF THE HEAD
IOHEXOL may be used to redefine diagnostic precision in areas of the brain which may not otherwise
have been satisfactorily visualized.
CT SCANNING OF THE BODY
IOHEXOL may be useful for enhancement of computed tomographic images for detection and
evaluation of lesions in the liver, pancreas, kidneys, aorta, mediastinum, pelvis, abdominal cavity,
and retroperitoneal space.
Enhancement of computed tomography with IOHEXOL may be of benefit in establishing diagnoses of
certain lesions in these sites with greater assurance than is possible with CT alone. In other cases,
the contrast agent may allow visualization of lesions not seen with CT alone (ie, tumor extension) or
may help to define suspicious lesions seen with unenhanced CT (ie, pancreatic cyst).
DIGITAL SUBTRACTION ANGIOGRAPHY
EXCRETORY UROGRAPHY
IOHEXOL 350 at a concentration of 350 mgI/mL is indicated for use in adults in excretory urography to
provide diagnostic contrast of the urinary tract.
-Oral/Body Cavity Use
VOIDING CYSTOURETHROGRAPHY (VCU)
IOHEXOL diluted to concentrations from 50 mgI/mL to 100 mgI/mL is indicated in children for voiding
cystourethrography. VCUs are often performed in conjunction with excretory urography.
ARTHROGRAPHY
IOHEXOL 350 at a concentration of 350 mgI/mL is indicated in radiography of the knee joint in adults.
4. Possible side effects
Adverse reactions following the use of IOHEXOL 350 are usually of mild to moderate severity. However, serious, life-threatening and fatal reactions, mostly of cardiovascular origin, have been associated with the administration of iodine-containing contrast media, including IOHEXOL. The injection of contrast media is frequently associated with the sensation of warmth and pain, especially in peripheral angiography; pain and warmth are less frequent and less severe with IOHEXOL than with many contrast media.
Cardiovascular System: Arrhythmias including PVCs and PACs (2%), angina/chest pain (1%), and hypotension (0.7%). Others including cardiac failure, asystole, bradycardia, tachycardia, and vasovagal reaction were reported with an individual incidence of 0.3% or less. In controlled clinical trials involving 1485 patients, one fatality occurred. A cause and effect relationship between this death and iohexol has not been established.
Nervous System: Vertigo (including dizziness and lightheadedness) (0.5%), pain (3%), vision abnormalities (including blurred vision and photomas) (2%), headache (2%), and taste perversion (1%). Others including anxiety, fever, motor and speech dysfunction, convulsion, paresthesia, somnolence, stiff neck, hemiparesis, syncope, shivering, transient ischemic attack, cerebral infarction, and nystagmus were reported, with an individual incidence of 0.3% or less.
Respiratory System: Dyspnea, rhinitis, coughing, and laryngitis, with an individual incidence of 0.2% or less.
Gastrointestinal System: Nausea (2%) and vomiting (0.7%). Others including diarrhea, dyspepsia, cramp, and dry mouth were reported, with an individual incidence of less than 0.1%.
Skin and Appendages: Urticaria (0.3%), purpura (0.1%), abscess (0.1%), and pruritus (0.1%).
Individual adverse reactions which occurred to a significantly greater extent for a specific procedure are uled under that indication.
DIRECTIONS FOR PROPER USE OF IOHEXOL PHARMACY BULK PACKAGE
• The transfer of IOHEXOL (Iohexol Injection) from the Pharmacy Bulk Package is restricted to a suitable work area, such as a laminar flow hood.
• The container closure may be penetrated only one time, utilizing a suitable transfer device and aseptic technique.
• The withdrawal of container spans should be accomplished without delay. However, should this not be possible, a maximum time of 8 hours from initial closure entry is permitted to complete fluid transfer operations. The container should not be removed from the aseptic area during the entire 8 hour period.
• The temperature of the container should not exceed 30℃, after the closure has been entered.
5. Storing IOHEXOL-REX
Do not freeze. IOHEXOL should be stored at 20-25℃; excursions permitted to 15-30℃, protected from light.