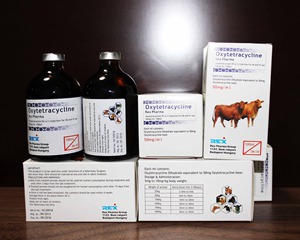
Oxytetracycline injection 50mg/ml
1. Product name: Oxytetracycline injection
2. Specification: 50mg/ml 100ml vial 5%
3. Package: 1 vial/box
4. Shelf life: 36 months
5.Registration dossiers are available.
1. Product name: Oxytetracycline injection
2. Specification: 50mg/ml 100ml vial 5%
3. Package: 1 vial/box
4. Shelf life: 36 months
5. Registration dossiers are available.
If you are interested in our products, please feel free to contact us.
1. COMPOSITION:
Each ml of OXYTETRACYCLINE injection contains: Oxytetracycline Dihydrate BP equivalent to 200mg of Oxytetracycline.
2. PROPERTIES AND ACTION:
OXYTETRACYCLINE injection contains Oxytetracycline which is a broad spectrum antimicrobial agent with bacteriostatic activity. The spectrum of action of Oxytetracycline encompasses a wide range of pathogens including chlamydia, mycoplasma, rickettsiae, spirochaetes and also many aerobic and anaerobic Gram-positive and Gram-negative bacteria and some protozoa. On parenteral administration, peak plasma levels are achieved in 4-8 hours making the preparations suitable for the treatment of acute infections.
On parenteral administration, peak plasma levels are achieved in 4-8 hours making the preparation suitable for the treatment of acute infections.
Oxytetracycline is taken up into sensitive bacterial cells by an active transport process where it binds reversibly to the 30s subunit of the ribosome, preventing the binding of aminoacyl transfer RNA and inhibiting protein synthesis and hence cell growth.
OXYTETRACYCLINE injection is specially formulated to provide persistent therapeutic blood levels for a prolonged period of time thus resulting in sustained antimicrobial activity for at least 4 consecutive days.
3. INDICATIONS:
OXYTETRACYCLINE injection is indicated in the treatment of a wide range of systemic, urinogenital, respiratory and local infections, including mastitis caused by or associated with pathogens sensitive to oxytetracycline in cattle, sheep, goats and pigs.
OXYTETRACYCLINE injection is used in the treatment and control of the following diseases in domestic animals.
Cattle:
Anaplasmosis, calf-diphtheria, actinobacillosis, foul-in-the-foot, pasteurellosis, pneumonia, heartwater, metritis, supportive therapy in mastitis, control of post-operative and post parturient infections, leptospirosis and actinomycosis.
OXYTETRACYCLINE injection is also used as supportive therapy in the treatment of East Coast Fever (ECF)
Sheep and Goats:
Foot-rot, mastitis, navel ill, pneumonia, metritis, chlamydiosis and contagious caprine pleuropneumonia (CCPP).
Pigs:
Navel ill, erysipelas and Mastitis Metritis Agalactiae (MMA) syndrome.
OXYTETRACYCLINE injection is also used in the treatment and control of non-specific conditions, including secondary bacterial infections which usually accompany viral diseases such as viral diseases such as viral pneumonia of pigs, and it has also proved to be effective in the control of infections that may occur following abdominal surgery.
4. DOSAGE AND ADMINISTRATION:
OXYTETRACYCLINE injection is administered by deep intramuscular (IM) injection at a dose of 1ml per 10kg body weight, which provides 20mg of oxytetracycline per kg body weight. One dose is usually sufficient for treatment, although it may be repeated if need be.
5. PRECAUTION AND WARNINGS:
1. OXYTETRACYCLINE injection is not recommended for use in horses, dogs and cats.
2. Once a vial has been broached the contents should be used within 4 weeks.
3. Milk from treated animals should not be used for human consumption until after 7 days from the last treatment.
4. Treated animals should not be slaughtered for human consumption until after 21 days from the last treatment.
5. The use of oxytetracycline preparation during the period of tooth development, including late pregnancy may lead to tooth discolouration
6.LEGAL CATEGORY:
Prescription Only Medicine (POM).
7. STORAGE CONDITION:
Store in a dry place below 30 . Protect from light. Keep all medicines out of reach of children.
. Protect from light. Keep all medicines out of reach of children.
8. PRESENTATION:
50ml and 100ml Vials.